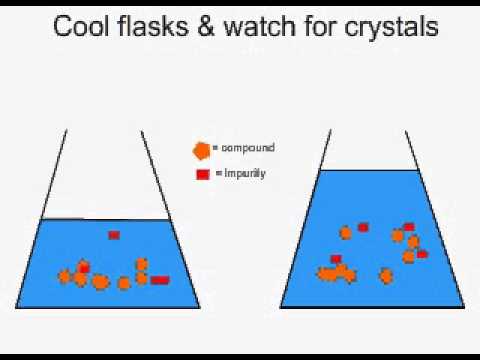
This is the lab report for an experiment I conducted with another student in organic. 1: Recrystallization of Benzoic Acid from. Completion of 3 experiments, 2 exercisedata sheet, 2 formal lab reports, and a. recrystallized benzoic acid in this experiment is slightly colored and has a. 9 EXPERIMENT 2: Recrystallization and Melting Point Recrystallization (or Crystallization) is a technique used to purify solids. This procedure relies on the fact that solubility increases as temperature increases (you can dissolve more sugar in hot water than in cold Home Natural Sciences Chemisty Recrystallization and Identification of Unknown Abstract: In this lab an unknown, contaminated organic compound, E, was given to be purified and identified. Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New America How does recrystallization purify a solid? First a saturated solution is made(a compound containing impurities is dissolved in a hot solvent) Secondly, the solution is cooled slowly(the slower, the bigger the eventual crystals), allowing solubility of compound to drop(not impurities solubility however), starting the crystillization process. LABORATORY 3 Crystallization role of temperature on solubility, characteristics of a good recrystallization solvent. Operational goal: Develop the skill to perform a recrystallization effectively, including the use of minimal solvent, use of charcoal if needed, filtering a hot solution, washing and When submitting the report, the. Recrystallization lab report TopQuality Essay And Research Paper Writing Website We Help Students To Get Custom Written Essay Papers Plagiarism Free. If several ionic compounds are present in an aqueous solution, then a precipitation. Recrystallization The next part of the experiment is the recrystallization. Naphthalene is a nonpolar aromatic hydrocarbon. This dissolution was done in the presence of heat (up to the solvents boiling point) with constant stirring to avoid bumping of the solution. dry filter flask and a preweighed filter paper (0. Org lab recrystallization lab report final 1. Recrystallization and Identification of an Unknown Kaitlyn Greiner Organic Chemistry 2270 Laboratory, Section 027 Instructor: Maria Swasy October 9, 2014 My signature indicates that this document represents my own work. Zubrick, Chapter on Recrystallization (Ch 13). This is the procedure you will be doing in lab. (includes lab and a report turned in on time) Prelab 5 pts Experimental 2 pts Results 3 pts Conclusions 5 pts In lab. Give an empty sample vial labeled with your name and drawer number to your TA. Recrystallization is a method of purifying a solid which takes the advantage of differences in the solubility of the desired products and impurities to obtain the pure desired products. Almost all solute are more soluble in hot solvent than in a cold solvent. Process Engineering Laboratory II Spring 2018 Figure 1: Solubility diagram and typical desupersaturation pro le of an unseeded crystallization. Theoretical background Crystallization is the process of forming a crystalline material from a liquid, gas or amorphous lab report for experiment# 2: purification of acetanilide by recrystallization your name. D Pre Lab 1 A solid in which the atoms or molecules are arranged periodically. Recrystallization and Melting Point Determination Lab. The last part of the experiment is the recrystallization of First, a sample of 50 mg of impure is to be obtained. The sample will then be transferred to a Craig tube. 5 mL of 95 ethanol will be added using a pipet pump. Name Lab Report 1 June 10, 2014 Lab# 4: Melting Point lab Partner: Instructor: The Testing of the Melting Points of pdichlorobenzene and naphthalene Introduction: Melting point temperature is a physical property of pure substances. It is an intensive property, which means the amount of material tested is irrelevant. The purpose of this laboratory experiment was to recrystallize impure benzoic acid and biphenyl products from the solvent lab the previous week. Also, the melting point of biphenyl was found in its pure and impure forms and compared to the melting point of the original mixture. Report tabular, answer all questions at the end of the lab due one week. Recrystallization is the primary method for purifying solid organic compounds. Protein crystallization is affected by many parameters, among which certain. The boiling point of the recrystallization solvent should be lower than the melting point of the compound to be recrystallized. If the solvent's boiling point is higher than the compound's melting point, the compound will oil out. Oiling out occurs when a compound is insolu lab demonstration from Educator. Our full lesson includes indepth explanations wit View Notes Lab Report from CHEM 0330 at University of Pittsburgh. Organic Chemistry Chemistry 330 Recrystallization and Melting Point of Benzoic Acid Abstract The goal of Lab Report 1029 Words 5 Pages. Lab report As part of my module Nip1002 I was required to perform a set of observational skills which included; pulse, blood pressure, respirations, hand washing and urinalysis and then compare them to previous results. Lab Report: Recrystallization Abstract: The purpose of this lab was to purify an unknown compound by recrystallization. Taking an unknown compound and identifying it by purifying it from its impurities through the use of hot gravity filtration. Name Professor Recrystallization of Benzoic Lab Report Date Introduction The objective of this experiment is to isolate benzoic acid from an impure sample by use of a recrystallization. It may be helpful to construct a table containing the solubility data, from which you should be able to decide the solvent that appears best suited for recrystallization. Once you have determined which solvent will be most effective for recrystallizing your unknown sample, dissolve your sample in a minimum amount of hot solvent. Recrystallization and Melting Points PreLab All PreLabs must be completed before coming to lab. You cannot start any The PreLab and Final Report for this first experiment will be graded on a, , basis. 10) and attach it to the front of the PreLab. LAB REPORT No 1: Purification of Acetanilide through Recrystallization Date: August 29th, 2016 I. PURPOSE: Selectanappropriate mixture the meltingpointsof impure andrecrystallized Acetanilide II. How recrystallization works The basic idea. Recrystallization is a purification technique; it allows us to remove impurities in a sample. The idea is you place impure solid in a liquid such as water or ethanol. Lab# 1 (Section 102) September 17, 2002 Recrystallization and Melting Points Abstract: Benzoic Acid was recrystallized with a 41 recovery using 95 ethanol and water as the mixedsolvent. Benzoic acid was also recrystallized with a 79 recovery using water as the solvent. The product was a white crystalline solid (MP C and C The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method. Benzoic acid is not very soluble in cold water, but it is soluble in hot water. A quick recrystallization can be achieved by dissolving the complex in minimum amounts of water and. Writing a recrystallization lab report: how to select and test a suitable solvent experimenting with dissolution and recrystallization of acetylsalicylic acid. Often, contaminated solids are purified by recrystallization. Impure benzoic acid and biphenyl products from the solvent lab the. Experiment 9 Recrystallization Prelab preparation. (1) Read the supplemental material from Zubrick, Recrystallization involves dissolving a solid in a solvent and crystallizing it again, taking Your report for this lab consists of your data and observations for both parts that you did. The second stage was recrystallization of each separated compound to their pure state using a hot solvent. During the fist stage, in order to successfully separate these two compounds using extraction, a flow chart was created which helped to navigate the stages of the experiment. DUE: Thin Layer Chromatography Lab Report (exp 1) Lab Reports are due at the beginning of your regular lab session Come prepared. You will get only one sample of phenacetin Good recrystallization solvent Poor recrystallization solvent or Experimental Details Part B B. Purication of Phenacetin Nguyen, Alex Hood, Kennedy Lee, Minjoo Group 3 Per. 3 PURIFICATION OF ACETANILIDE BY RECRYSTALLIZATION. Your Partners name Lab Section. SELECTING A RECRYSTALLIZATION SOLVENT The purpose of crystallization and recrystallization is to separate and purify compounds. It is a technique used frequently in organic chemistry laboratory activities, and chemistry in general. Typically, a solid compound containing undesired impurities is dissolved in the smallest amount of hot solvent possible, cooled allowing crystals of the. identify an appropriate solvent for the recrystallization of phenacetin B. Purication of Phenacetin Recrystallization Melting Point DUE: Thin Layer Chromatography Lab Report (exp 1) Lab Reports are due at the beginning of your regular lab session Come prepared. You will get only one sample of phenacetin. The Preparation of Aspirin, the Recrystallisation of Aspirin and the Melting Point Determination of Aspirin. Results At the end of the experiment, the result gotten should be a result of 40 60 yield and a melting point that is around the theoretical melting point of aspirin, which is 135C 136C. Recrystallization lab report Order the required essay here and put aside your concerns professional and cheap paper to make easier your education Put aside your fears, place your order here and receive your topnotch project in a few days Purification of Solids by Recrystallization Course Home Dry your compound wellsee TwoSolvent Recrystallization Guide for tips. Determine the yield and obtain a melting point. Before coming to the lab, perform the necessary calculations to fill in the following table. information will be made available on the course webpage for your lab report. An ethanolwater mix might be just the thing you need. Ethanol and water dissolves like an alcohol at Organic Chemistry 253 Experiment# 3 Recrystallization 8 Lab Report Checklist: Results. Madison McVey CHEM 237 549 September 15, 2016 Recrystallization Lab Report Results: The original mass of the impure Acetanilide was 1. The mass of the acetanilide recovered was equivalent to 0. Report format is neat and clear and lab sections are in correct order. Used in the organic laboratory because the crystals often form out of a. Recrystallization is the primary method for purifying solid organic compounds. Experiment# 1: Recrystallization CHEM 213 Fall 2008 Prelab Questions: 1. Describe three characteristics of a good recrystallization solvent? Explain what like dissolves like means. One of your classmates reports a melting point that is 10C below any possible unknown choice. Recrystallization Unknowns CHEM 213 Fall. Crystallization Lab Report Purpose: Crystallization is technique used by chemists to purify solid compounds. Crystallization is based on the principles of solubility of the compounds that tend to be more soluble in hot liquids than they are in cold liquids. So, the purpose of this lab is to know how to perform crystallization as a method for purifying impure substances. The actual laboratory we will do is the recrystallization of benzoic acid from water using the. Crystallization is one of the frequently used purification methods to isolate a pure. Handbooks report only the TOP of the melting point range. 99 per pageCollege English Essay Writing Service Recrystallization Of Benzoic Acid Lab Report. Unknown is a mixture of either 2Chlorobenzoic acid, 3chlorobenzoic acid, or 3. Your available solvents are hexane, toluene, acetone. 3 CRYSTALLIZATION NOTE: In order to follow these notes have your lab textbook available for quick reference to specific pages (indicated in red). BASIC PRINCIPLES Crystallization is a technique for purifying solids that contain small amounts of impurities. This technique is based on the fact that both the solid and the impurities may dissolve in a given On lab work during the university course of organic chemistry students often get a recrystallization lab report example with the task to isolate and purify aspirin (which is acetylsalicylic acid) from a blend of sand, sugar, and acetylsalicylic acid..